Advanced therapies, particularly cell and gene therapies, are ushering in a new era of personalized medicine, offering hope for patients with previously untreatable diseases. These cutting-edge approaches harness the power of living cells and genetic material to address the root causes of illnesses, fundamentally transforming how we perceive and treat complex medical conditions.
Anemocyte supports pDNA and mRNA manufacturing — critical components in the advanced therapy landscape, shaping the future of cell and gene therapies.
- Next-Generation Gene Editing Technologies
The advent of gene-editing tools like CRISPR-Cas9 revolutionized molecular biology, enabling precise genetic modifications. Recent advancements, such as prime editing and base editing, have refined this technology, offering unprecedented accuracy and reduced off-target effects. These tools are opening new avenues for treating genetic disorders like sickle cell anemia, cystic fibrosis, and even rare monogenic diseases.
Additionally, emerging platforms such as CRISPR-associated transposases and RNA-guided gene editors are expanding the scope of what gene therapy can achieve, moving closer to curing diseases at their genetic roots. Complementing these efforts, the availability of high-quality plasmid DNA (pDNA) and messenger RNA (mRNA) manufacturing capabilities—offered by industry leaders like Anemocyte—is critical for enabling gene editing platforms and therapeutic development.
- Allogeneic Cell Therapies
Autologous therapies, derived from a patient’s own cells, have been a cornerstone of cell therapy development. However, the shift toward allogeneic approaches—using cells from healthy donors—promises scalability and accessibility. Allogeneic therapies, often termed “off-the-shelf” solutions, are being developed for conditions like haematological cancers and autoimmune diseases.
For example, engineered T-cells, such as CAR-T therapies, are being optimized for allogeneic use, enabling faster and more cost-effective treatments while reducing manufacturing challenges. The scalability of these solutions is further supported by advancements in pDNA production, which underpins the genetic engineering processes required for these therapies.
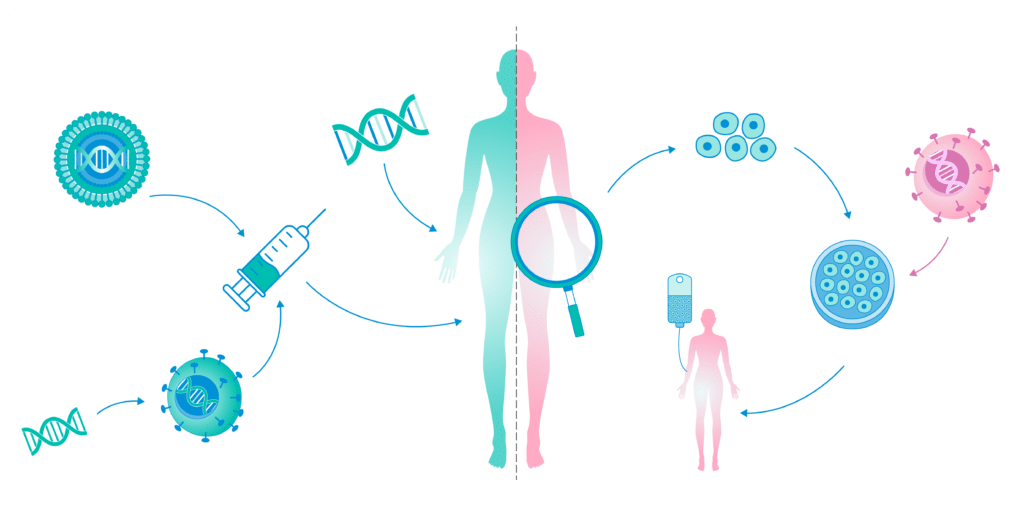
- Gene Therapies for Rare Diseases
The potential of gene therapies for rare diseases is becoming increasingly apparent. Advances in adeno-associated virus (AAV) vector design have enabled more effective delivery of therapeutic genes to target cells. New developments in lentiviral and non-viral delivery systems are addressing previous limitations, such as immune responses and limited payload capacities.
The approval of therapies like Zolgensma for spinal muscular atrophy exemplifies the impact of gene therapies in transforming the lives of patients with rare genetic disorders. Companies like Anemocyte play a crucial role in this space by supporting the manufacturing of pDNA and mRNA, which are essential for developing and scaling these therapeutic solutions.
- Ex Vivo vs. In Vivo Approaches
In the past, ex vivo gene therapies—where cells are modified outside the body and reintroduced—dominated the field. However, in vivo therapies, where genetic material is delivered directly into the patient, are gaining momentum. This paradigm shift reduces complexity and broadens the range of treatable conditions.
Technological innovations in nanoparticle delivery systems and the use of tissue-specific promoters are enhancing the safety and efficacy of in vivo therapies, paving the way for widespread adoption. The production of high-quality pDNA and mRNA, as facilitated by Anemocyte, is fundamental to these in vivo applications, ensuring reliable and scalable therapeutic solutions.
- AI and Machine Learning in Therapy Development
Artificial intelligence (AI) and machine learning are revolutionizing the discovery and optimization of advanced therapies. These technologies accelerate the identification of viable gene-editing targets, predict off-target effects, and streamline the development of delivery vectors.
For example, AI-driven models are being used to analyze patient genomes for personalized treatment plans, ensuring that therapies are not only effective but also tailored to individual needs.
- Addressing Manufacturing Challenges
The scalability of cell and gene therapies has long been a hurdle, but emerging innovations in bioprocessing are offering solutions. Technologies like single-use bioreactors, automated manufacturing platforms, and advanced cryopreservation techniques are enabling more efficient production pipelines.
Additionally, the manufacturing of critical raw materials, such as pDNA and mRNA, is being optimized to meet the growing demand for advanced therapies. Anemocyte’s expertise in this area ensures high-quality production, contributing to faster and more reliable therapeutic development. Furthermore, evolving regulatory frameworks are accommodating the unique nature of these therapies, facilitating faster approval processes while maintaining rigorous safety standards.
For example, AI-driven models are being used to analyze patient genomes for personalized treatment plans, ensuring that therapies are not only effective but also tailored to individual needs.
Conclusion: A New Horizon in Medicine
The future of cell and gene therapies is bright, with scientific advancements poised to redefine the boundaries of medicine. As we continue to overcome technological, logistical, and regulatory challenges, these therapies promise to deliver curative solutions for a growing number of patients worldwide.
At Anemocyte, we are proud to contribute to this transformative field, supporting the development and scalability of advanced therapies through our expertise in pDNA and mRNA manufacturing. By enabling these critical components, we are helping to shape the future of healthcare. The journey has just begun, and the possibilities are limitless.