Anemocyte, a leading biotech company specializing in the development and manufacturing of plasmid DNA (pDNA) and mRNA, is driving a transformative shift in genetic medicine. The company’s advanced manufacturing capabilities are empowering biotech and pharmaceutical companies developing non-viral genetic modification techniques, offering safer and more adaptable alternatives to traditional viral vector methods. These innovations are unlocking groundbreaking possibilities in cell and gene therapies (CGT), setting the stage for a new era of safer and more effective treatments.
The Rise of Non-Viral Genetic Modification
Gene therapy has historically relied on viral vectors to deliver genetic material into cells. However, concerns over safety, immunogenicity, scalability, and long-term risks have fueled the rise of non-viral techniques. These approaches are reshaping the field of genetic medicine by addressing these challenges and offering innovative solutions.
Innovative Non-Viral Delivery Systems
Non-viral technologies are delivery systems that avoid the risks associated with viral vectors, here some examples:
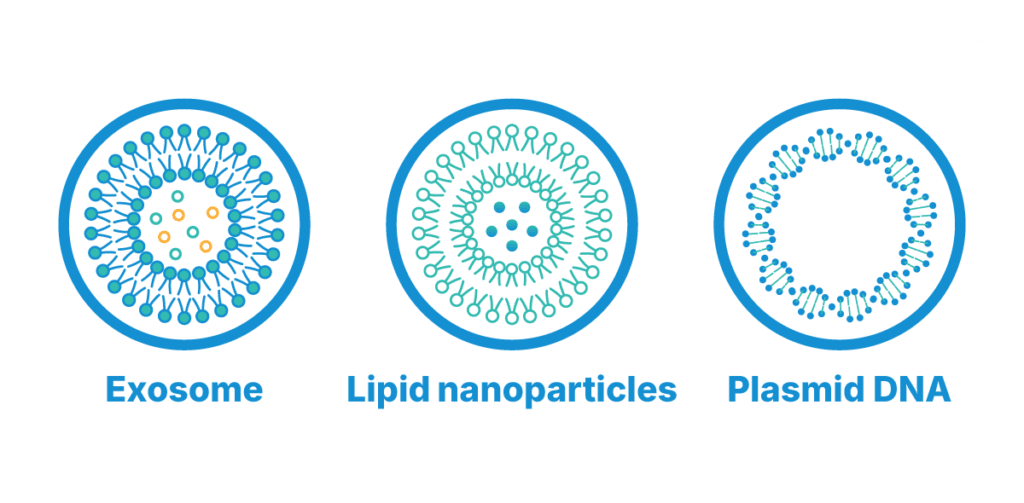
- Lipid Nanoparticles (LNPs): Advanced carriers that gained prominence with mRNA vaccines, LNPs demonstrate exceptional efficiency in delivering genetic material without triggering immune reactions. Their customizable surfaces enable precise targeting, enhancing therapeutic outcomes.
- Plasmid DNA (pDNA): A key component in non-viral gene therapy, pDNA serves as a versatile and efficient tool for genetic modification, supporting both transient and permanent genetic expression.
- mRNA Delivery: mRNA, delivered using non-viral systems like LNPs or hybrid methods, allows for direct protein expression with reduced risk of long-term genomic integration.
- CRISPR-Cas Ribonucleoproteins (RNPs): This genome-editing tool eliminates the need for viral carriers, offering highly precise modifications without permanent changes to the genome.
- Extracellular Vesicles (EVs): Natural particles used by cells for communication, EVs are being explored for their ability to safely and efficiently deliver genetic material with minimal immunogenicity.
These techniques collectively represent a paradigm shift, addressing critical safety concerns while offering scalable and adaptable solutions for a range of therapeutic applications.
Anemocyte's Leadership in pDNA and mRNA Manufacturing
As a leading developer and manufacturer of pDNA and mRNA, Anemocyte plays a pivotal role in enabling non-viral genetic modification techniques. The company’s state-of-the-art facilities and expertise ensure high-quality production and scalability for advanced therapies.
A Safer Future for Gene Therapy
The innovations in non-viral genetic modification, driven by advanced materials like pDNA and mRNA, signal a safer and more versatile future for gene therapy. By addressing safety concerns, improving scalability, and enabling precise targeting, these techniques are poised to replace viral vectors in numerous applications, making therapies safer and more accessible for patients worldwide.
At Anemocyte, we are proud to be at the forefront of this transformation. Our expertise in pDNA and mRNA manufacturing positions us as a critical partner for companies pioneering safer and more effective therapies.
About Anemocyte
Anemocyte is a global leader in the development and manufacturing of pDNA and mRNA for advanced therapies. With a strong commitment to safety, innovation, and patient-centered solutions, Anemocyte is transforming the field of cell and gene therapy, empowering biotech companies to deliver groundbreaking treatments to patients worldwide.
For more information, visit our website at anemocyte.com