In recent years, mRNA technology has emerged as a transformative force in the biopharmaceutical industry, enabling groundbreaking therapies and vaccines, including those for COVID-19. As the field expands, so do the challenges associated with the efficient and scalable production of mRNA. This article explores the obstacles in mRNA synthesis and purification and highlights cutting-edge solutions—including Anemocyte’s contributions—driving innovation in this space.
The Importance of mRNA Production
mRNA (messenger RNA) serves as a transient template for protein synthesis in cells, making it a versatile platform for therapeutic applications. From personalized cancer vaccines to gene therapies, the ability to produce high-purity, large-scale mRNA efficiently is critical for meeting the demands of a rapidly growing market.
However, producing mRNA involves intricate processes that require precise optimization to ensure quality, efficacy, and scalability. Each step—from transcription to purification—poses unique challenges, prompting researchers and manufacturers to continuously seek innovative solutions. Companies like Anemocyte are playing a pivotal role in overcoming these hurdles through advanced technologies and expertise.
Challenges in mRNA Production
- Efficiency of In Vitro Transcription (IVT)
In vitro transcription, the cornerstone of mRNA synthesis, involves the enzymatic transcription of a DNA template. Despite its widespread use, IVT faces several efficiency-related issues:
- Yield Limitation: High-yield production is crucial to meet clinical and commercial demands, but enzyme inefficiencies and template design can limit output.
- Unwanted Byproducts: IVT often generates incomplete or aberrant transcripts, as well as by-products which must be removed to maintain product integrity.
- Scalability: Scaling up the IVT process without compromising quality remains a significant hurdle.
- Capping Efficiency and Stability
For mRNA to be biologically active and stable, a 5′ cap structure is essential. However:
- Inefficiencies in Capping: Co- transcriptional and enzymatic capping processes can be inefficient, leading to incomplete capping.
- Degradation and Immune Response: Uncapped mRNA is rapidly degraded and can trigger immune responses, reducing therapeutic efficacy.
- Purification Bottlenecks
Purification is critical to remove impurities such as enzymes, DNA templates, and unwanted transcripts. However:
- Stringent Purity Requirements: Traditional chromatography methods can struggle to balance high yield with ultra-high purity essential for clinical applications.
- Immunogenic Impurities: Impurities at the ppm level can trigger adverse immune responses, necessitating advanced purification techniques.
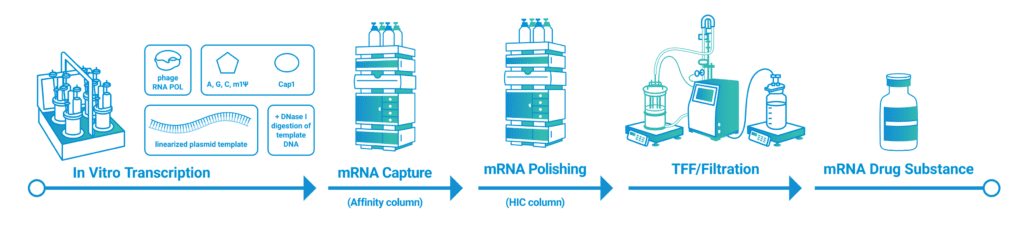
Cutting-Edge Solutions
Recent advancements in technology and process development are revolutionizing mRNA production. Anemocyte is at the forefront of these innovations.
Advanced Enzymes and Optimized IVT Systems
High-performance enzymes and optimized IVT conditions are enhancing transcription efficiency and yield:
- Polymerases: Engineered enzymes, reduce byproduct formation and improve scalability.
- Template Engineering: Tailored templates enable more efficient transcript elongation, further boosting productivity.
Next-Generation Capping Technologies
Innovations in capping methods have significantly improved cap incorporation efficiency:
- Co-Transcriptional Capping: This method ensures efficient and uniform cap addition.
- Modified Enzymatic Systems: Advanced enzymatic processes enhance stability and reduce the generation of aberrantly capped molecules.
Novel Purification Techniques
Addressing the challenges of yield and purity, Anemocyte integrates state-of-the-art purification methods into its mRNA production workflows:
- Membrane Chromatography: Faster and more scalable than traditional methods, membrane chromatography reduces processing time while maintaining stringent purity standards.
- Affinity Resins: Specialized resins designed to target specific mRNA features improve impurity removal and recovery rates.
- Monolithic columns: requires fewer unit operations and assure high flow rate and binding capacity while maintaining the ability to remove the impurities
- Nanofiltration: Cutting-edge nanofiltration technologies ensure the removal of viral particles and contaminants while preserving mRNA integrity.
Future Directions
The future of mRNA production is promising, with ongoing efforts to make the process faster, more cost-effective, and sustainable. Key trends include:
- Synthetic Biology: Designing more efficient templates and enzymes.
- Closed-Loop Systems: Minimizing contamination risks and enhancing process reliability.
- Continuous Manufacturing: Streamlining production with advanced analytical tools for consistent quality control.
Anemocyte is committed to driving these advancements. Through collaboration and investment in next-generation technologies, the company is helping to unlock new applications in personalized medicine and beyond.
Conclusion
As mRNA technology continues to revolutionize medicine, overcoming production challenges is paramount. Advancements in synthesis and purification methods—fueled by innovators like Anemocyte—are enabling manufacturers to scale up operations while maintaining the highest quality standards. With continued innovation, the future of mRNA production is poised to unlock new therapeutic possibilities, transforming healthcare on a global scale.