
Introduction
In the realm of gene therapy, the production of viral vectors is a critical step for delivering therapeutic genes to target cells. Two commonly used vectors are lentiviruses and adeno-associated viruses (AAV). Each of these vectors requires specific plasmids for their production, which ensure efficient vector generation and safe delivery of the gene of interest (GOI). This article will delve into the plasmids involved in the manufacturing of lentiviruses and AAVs, highlighting the traditional systems and innovative advancements that have streamlined the production process.
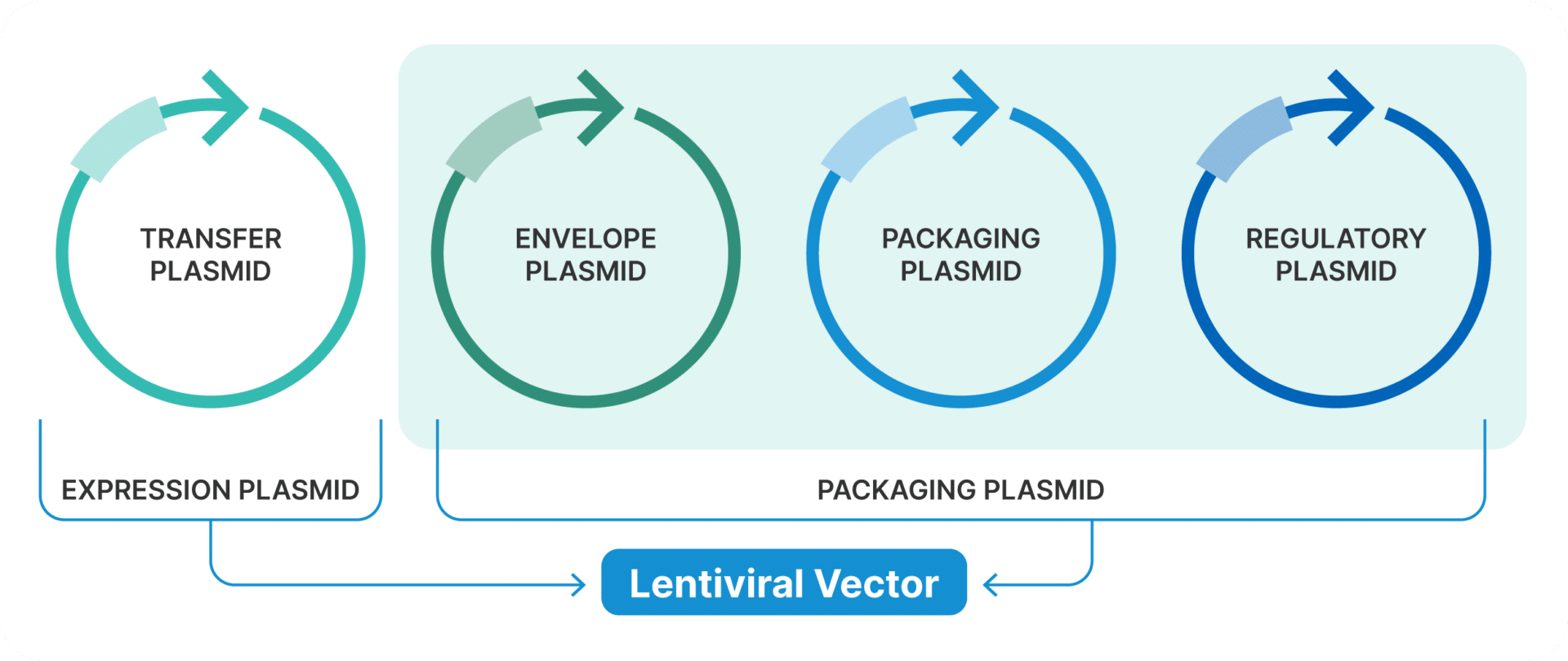
Lentivirus Plasmids
Lentiviral vectors are complex to produce, typically involving three to four plasmids that each serve distinct functions:
Transfer Plasmid
The transfer plasmid is the cornerstone of lentiviral vector production. It contains the GOI flanked by long terminal repeats (LTRs), which are necessary for the integration of the gene into the host genome. This plasmid also includes a promoter to drive the expression of the GOI once inside the host cell.
Packaging Plasmid
The packaging plasmid supplies the essential viral proteins, gag and pol, required for the assembly and replication of the virion. Importantly, this plasmid does not contain sequences needed for viral particle production, thus ensuring safety by preventing the creation of replication-competent lentivirus (RCL).
Envelope Plasmid
The envelope plasmid provides the envelope glycoprotein, commonly derived from the vesicular stomatitis virus (VSV-G). This glycoprotein enables the virus to infect a broad range of cell types, enhancing the versatility of the lentiviral vector.
Regulatory Plasmids (Optional)
In some production systems, additional regulatory plasmids are used. These may contain elements that enhance viral production or gene expression, thereby optimizing the overall efficiency of the vector production process.
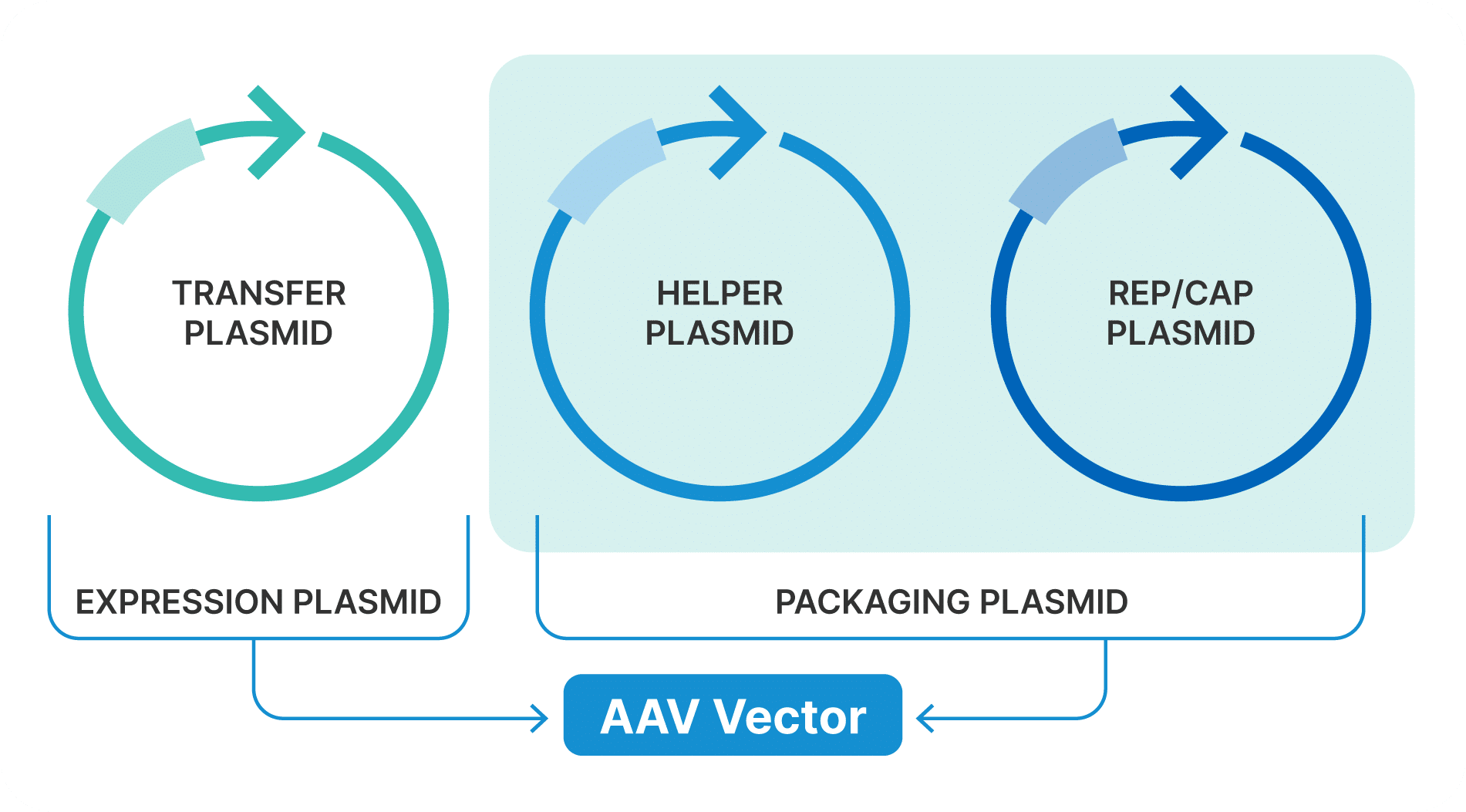
AAV Plasmids
AAV vector production typically utilizes either a triple-plasmid or dual-plasmid system:
Transfer Plasmid
Similar to the lentiviral transfer plasmid, the AAV transfer plasmid contains the GOI flanked by inverted terminal repeats (ITRs). These ITRs are crucial for the replication and packaging of the AAV vector genome.
RepCap Plasmid
The RepCap plasmid provides the AAV Rep and Cap genes, essential for the replication and encapsidation of the viral genome. The Rep genes facilitate the replication of the AAV genome, while the Cap genes encode the viral capsid proteins necessary for packaging the viral particles.
Helper Plasmid
The helper plasmid supplies necessary adenoviral functions (E1A, E1B, E2A, E4orf6, and VA RNA) that support AAV replication and packaging. This plasmid mimics the helper virus functions without producing infectious adenovirus particles, thus maintaining safety.
Innovative Systems in AAV Production
To enhance the efficiency and simplicity of AAV production, innovative systems have been developed, such as the dual-plasmid system and the pDG system:
Dual-Plasmid System
This system combines components of the traditional three plasmids into two plasmids, simplifying the production process. For example, one plasmid may include both RepCap and helper functions, while the other carries the GOI-ITR sequence. This consolidation can enhance efficiency and yield by optimizing the arrangement of Rep, Cap, and helper sequences.
pDG System
The pDG system is a specific type of dual-plasmid system that combines RepCap and adenoviral helper functions on one plasmid, paired with a separate GOI-ITR plasmid. This approach reduces the number of plasmids required for AAV production, potentially improving yield and streamlining the overall process.
Conclusion
Both lentiviral and AAV production systems rely on carefully designed plasmids that provide the essential genetic elements for vector production, packaging, and delivery. Innovations in plasmid design, such as the development of dual-plasmid systems, aim to enhance the efficiency and scalability of viral vector manufacturing. These advancements are crucial for advancing gene therapy applications, making treatments more effective and accessible.
In summary, understanding the roles and innovations of plasmids in lentiviral and AAV production is fundamental for optimizing gene therapy processes and achieving successful therapeutic outcomes.