
What’s plasmid DNA?
A plasmid is a circular double strand DNA that exists separately from the chromosomal DNA within a cell. Plasmids are commonly found in bacteria, where they replicate independently of the bacterial chromosome. These plasmids often carry genes that provide advantageous traits, such as antibiotic resistance.
Plasmids are versatile and essential tools in cell and gene therapies due to their ease of use, safety profile, and effectiveness in delivering therapeutic genes. Engineered plasmids can carry therapeutic genes to target cells, which can then express the therapeutic protein. Their application spans a wide range of diseases, offering the potential to correct genetic defects at their source. Additionally, plasmids can be produced in large quantities, making them advantageous for scaling up gene therapy treatments for clinical use.
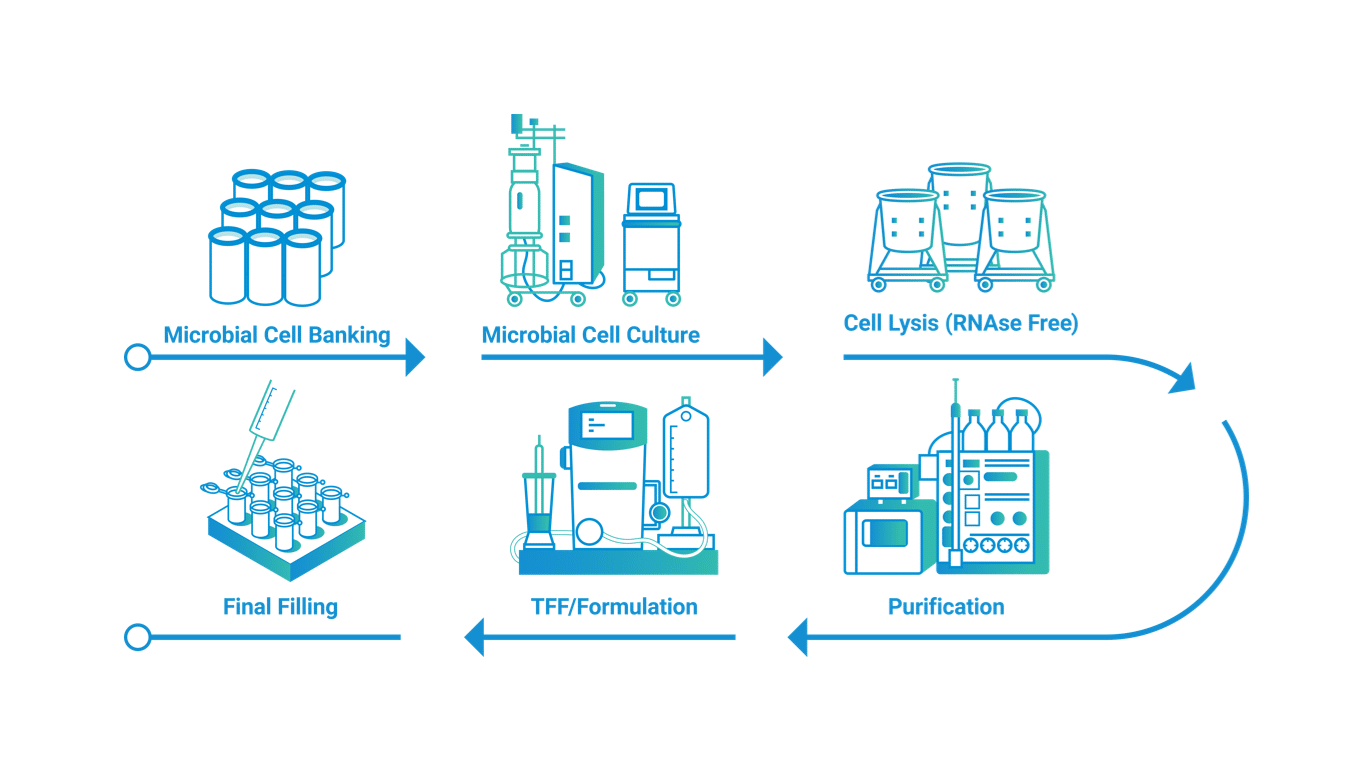
Manufacturing Process of plasmid DNA
Anemocyte has developed a plasmid DNA (pDNA) manufacturing platform to support the needs of the cell and gene therapy field. Anemocyte’s platform guarantees flexibility for multiple productions, accommodating a range of production scales from a few milligrams to multiple grams of plasmids. The dedicated production facility features classified clean rooms for different production phases, ensuring product segregation and avoiding cross-contamination.
Why Do We Need an MCB?
Plasmid DNA manufacturing at Anemocyte begins with the production of a Master Cell Bank (MCB). This process includes a preliminary phase using different E. coli strains aiming to address parameters and yield to enhance plasmid productivity. This preliminary phase is crucial for subsequent steps, leading to the production of an MCB that supports pDNA production for clinical and commercial phases. This feature allows the customer to streamline timelines and reduce variability among different batches.
Highly specialized competences in pDNA manufacturing
The MCB is used for the subsequent upstream phase, with production capacities ranging from 5L to 200L ensuring maximum production flexibility based on customer requirements. Highly specialized competences in pDNA manufacturing support a possible optimization during the fermentation step for microbial growth and plasmid production. Together with this, Anemocyte’s production team focuses on the continuous improvement of upstream processes, increasing efficiency and effectiveness. This approach ensures the company remains up-to-date with the latest market techniques while bringing innovation to the process itself.